The hype and hope of new food allergy treatments - Prof Perrett writes for Nature Medicine
- Published
- Monday, April 22, 2024 - 7:00 PM
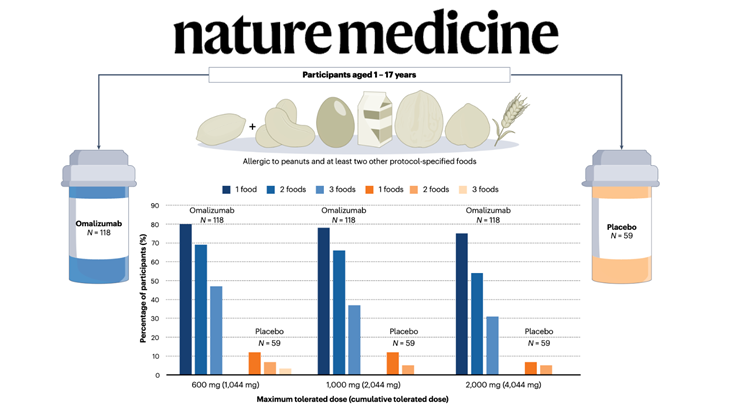
Food allergy treatment is undergoing a paradigm shift with new therapies emerging, including the recent FDA approval of omalizumab — but without evidence of disease modification and with uncertain quality-of-life improvement, it may not be a panacea for all.
In a recent issue of The New England Journal of Medicine, Wood et al. report results of the first stage (of three) of the OUtMATCH trial, which evaluated the monoclonal antibody omalizumab (anti-IgE) as monotherapy in children and adults with multiple food allergies.
On the basis of these results, the US Food and Drug Administration (FDA) approved the expanded use of omalizumab in children (from 1 year of age) and adults with IgE-mediated food allergy, making it the first medication approved to help reduce the risk of severe allergic reactions across multiple foods.
Professor Kirsten Perrett, National Allergy Centre of Excellence Director and Murdoch Children's Research Institute Population Allergy Group Lead, writes for Nature Medicine about the hype and hope of new food allergy treatments.
Read more in Nature Medicine






